DETECTING PROTEIN PHOSPORYLATION AND CELL SIGNALING BY FLOW CITOMETRY
Young Researcher's Corner - Autumn 2011, by Sara Ciullini Mannurita
Phosphorylation of proteins is an important reversible regulatory mechanism of post translational modifications that occurs in both prokaryotic and eukaryotic organisms due to enzymes called kinases (phosphorylation) and phosphatases (dephosphorylation) that regulate intracellular protein phosphorylation in many different cell signaling pathways (T and B cell signaling, those regulating apoptosis, growth and cell cycle control, those involved with cytokine, chemokine, and stress responses). Reversible phosphorylation results in a conformational change in the structure of many proteins that become activated or deactivated. In eukaryotic proteins phosphorylation usually occurs on serine, threonine, tyrosine and histidine residues. The addition of a phosphate group (-PO32-) to a polar R group of an amino acid residue introduce a conformational change in the structure of the protein turning a hydrophobic portion into a polar and hydrophilic portion of molecule that interact with other hydrophobic and hydrophilic residues in the protein. Upon the deactivating signal, the protein switches back to its original conformation.
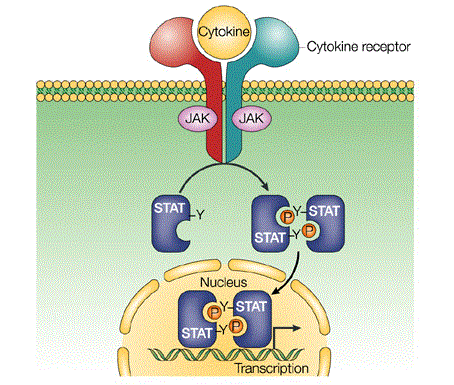
Janus tyrosine kinase (JAK)-Signal Transducers and Activators of Transcription (STAT) signaling pathway is one of the major cell signaling mechanism and it is important in transmission of information from chemical signals outside of the cell, through the cell membrane, and into gene promoters on the DNA in the cell nucleus causing DNA transcription and activity in the cell.
Signaling through the JAK/STAT pathway begins at the cell membrane when specific cytokines bind their target receptor. This triggers the autophosphorylation on tyrosines of non-covalently attached JAK kinases, which also phosphorylate signature tyrosine residues in the intracellular receptor tails thus providing sites for interaction with phosphotyrosine-binding SH2 domains located on STAT proteins. The bound STAT monomers are themselves phosphorylated by JAK on a single tyrosine residue at their COOH terminus inducing the detachment from their receptor docking sites and the formation of high avidity reciprocal homo- or heterodimers. This sequence of events (STAT activation) triggers the accumulation of STAT dimers in the nucleus where they can bind to palindromic DNA recognition sites (GAS) and directly induce transcription (Fig. 1).
In human there are seven STATs (STAT1, -2,-3, -4, -5a, -5b, -6) and they are expressed in multiple cell types, are activated by many growth factors such as growth hormone, prolactin and cytokines, and paticipate in a diverse set of biological activities. They are greatly evolutionary conserved and their mutations, disruption or dysregulated functionality (which is usually inherited or acquired genetic defects) can result in cancers and immune deficiency syndromes (i.e STAT1 deficiency, hyper-IgE syndrome, STA5b deficiency).
In the past phospho protein detection has been performed with techniques using radioactivity as radiometric kinase assays and phosphoaminoacid labeling. However, the advent of phospho specific antibodies has facilitated the use of more straightforward techniques such as western blotting, ELISA, immunoprecipitation, and immunofluorescence microscopy. These techniques, however, have several shortcomings as they require a relatively large amount of sample, are time consuming, do not produce truly quantitative results and are not conducive to multiparameter analysis.
Flow cytometry is advantageous because it allows rapid, quantitative and single cell analysis. Proteins can be detected in a specific cell type within a heterogeneous population via cell surface marker phenotyping without the need to physically separate the cells. In this way, a small, rare population of cells may be analyzed without concern for cell loss or altered protein expression that may occur during a cell-sorting process.
Detection of phospho proteins by flow cytometry requires that the protein is stable and accessible to the antibody. Cells or whole blood can be used for the analysis. First of all the sample is stimulated with specific mytogens depending on the pathway of cell signaling where the phospoprotein is involved in (i.e. IL2 activate phosphorylation of STAT5). Cells stimulation is a critical step of this process because phosporylation is influenced by the type of cells and stumuli, the time of stimulation and concetration of mytogens. Immediately after stimulation cells are fixed with formaldehyde or paraformaldehyde to cross-link the phospho proteins and stabilize them for analysis. Cell staining before fixation is not recommended because it could activate cells in an unspecific way alterating the phospho protein phosporylation state. The fixed cells must be permeabilized to allow the entry of phospho-specific antibodies into the cell. Different permeabilization techniques are often useful for various subcellular locations. A mild detergent will allow the detection of cytoplasmic proteins, while alcohol may be required for antibody access to nuclear proteins. Alcohol permeabilization may also enhance phospho protein detection using peptide specific antibodies due to the denaturing property of alcohol. Then cells are stained at the same time with fluorophore-coniugated antibodies binding the phospho protein and the superficial markers, washed, acquired with the flow cytometer and analyzed as a classical multiparametric analysis. The positive percentages represent the quantity of cells that show phosporilation of the specific protein or the percetages of activated cells responding to the specific stimulation. The pecentage of positivity needs to be evaluated compared with the not stimulated negative control.
This method is a powerful tool to study downstream signaling in T and B cells following respectively TCR and BCR activation and in response to specific stimuli, evaluating which signaling cascates is activated, the kinetics of signaling and the downstream target that are transcribed. Moreover, comparing diseased cells to healty samples, it is possible to identify aberrant signaling, changes in key phosphotyrosine residues and analyze the effect of a mutation in the phospho protein.
References
Bonilla LE, Means GD, Lee KA and Patterson SD. The evolution of tools for protein phosphorylation site analysis: from discovery to clinical application, BioTechniques (2008) 44:671-679.
Liu KD, Gaffen SL, Goldsmith MA. JAK/STAT signaling by cytokine receptors, Curr Opin Immunol. (1998), 10(3):271-8.
Krutzik PO, Irish JM, Nolan GP, Perez OD. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: techniques and clinical applications. Clin Immunol (2004), 110(3):206-21.
Notarangelo LD. Primary immunodeficiencies, J Allergy Clin Immunol (2010), 125:S182-94.
Shuai K and Liu B. Regulation of JAK–STAT signalling in the immune system, Nature Reviews Immunology, (2003), 3, 900-911.
Sara Ciullini Mannurita